Abstract
BACKGROUND: Adoptive transfer of in vitro expanded autologous and allogeneic virus specific T (VST) cells has been successfully used to prevent and treat EBV viral reactivation in transplant patients and aggressive EBV-driven cancers such as post-transplant lymphoproliferative disease (PTLD), nasopharyngeal carcinoma, and extranodal NK/T-cell lymphoma. Due to the easy accessibility of peripheral blood, VST cell products are universally generated from circulating T cells. However, the T cells in circulation represent only a minor fraction of T cells in the body with most residing in tissue sites, particularly lymph nodes. Recent animal data suggest that unique T cell populations that sustain memory responses to chronic viral infections exclusively reside in lymph nodes. The efficacy of using lymph node-derived T cells for adoptive cellular therapy has not been reported.
AIMS: To assess the feasibility of generating VST cells from human lymph nodes using our clinically-compatible strategy and to test the ability of T cells derived from peripheral lymph nodes to expand in response to EBV-derived viral antigens and display functionality compared to T cells derived from blood.
METHODS: Human blood and lymphoid tissues were obtained from brain dead organ donors at the time of organ procurement for transplantation through an approved protocol with LiveOnNY. Human blood was also obtained from healthy volunteers through an IRB approved protocol. Donors were cancer free, EBV seropositive, and negative for hepatitis B, C and HIV. Lymph nodes were isolated in sterile fashion, enzymatically and mechanically digested to a single cell suspension. Overlapping 15 mer peptide pools (pepmixes) of EBV latency viral antigens EBNA1 and LMP1 (JPT, Berlin, Germany) were used for expansion and restimulation. T cells were isolated by fluorescence activated cell sorting and stimulated with peptide pulsed irradiated mononuclear cells from blood (healthy donors) or spleen (organ donors), followed by 14-day culture in IL-7 and 15 (10 ng/mL) with addition of IL-2 (20 IU/mL) starting on day +3. Expanded T cells were then rested overnight and restimulated with individual pepmixes for 6 hours followed by surface marker and intracellular cytokine staining to evaluate differentiation state and function.
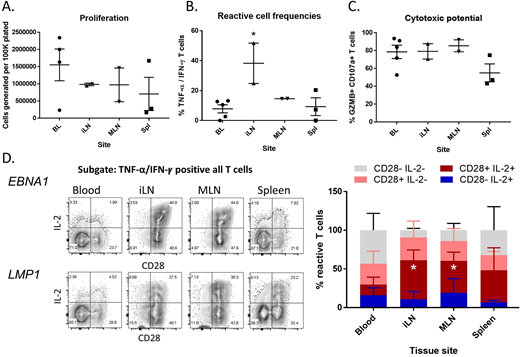
RESULTS: T cells from lymph node, blood and spleen displayed comparable levels of in vitro expansion (Fig. 1A). Compared to blood, there was increased EBNA1 reactive cell frequency (TNF-α/IFN-ꝩ positive) in the lymph node derived T cell cultures (Fig. 1B). VST cells were predominately CD8 from blood (56 ± 15%) and lymph node (86 ± 3.8%) but not spleen (24 ± 6.4%). One donor in this cohort displayed significant reactivity for LMP1. Both blood and lymph node derived VST cells were uniformly positive for granzyme B and the degranulation marker CD107a (Fig. 1C). Remarkably, the lymph node derived VST cells displayed markedly enhanced polyfunctionality with robust secretion of IL-2, as well as increased surface expression of the co-stimulatory molecule CD28 with 33±3.6% displaying strong co-expression of both molecules compared to 8.1±2.7% of those derived from blood (Fig. 1D).
CONCLUSION: These results suggest that it is feasible to generate highly-reactive EBV-specific T cells from human lymph node tissue using the methodology compatible with good manufacturing practice (GMP). In contrast to VST cells derived from peripheral blood, increased expression of CD28 and IL-2 on lymph node derived EBV reactive cells may indicate a superior capacity to survive, expand in vivo and eradicate EBV-driven disease upon adoptive transfer.
Figure 1. Characterization of lymph node derived EBV reactive T cells.
A) Expanded T cells from Blood (BL), iliac lymph node (iLN), mesenteric lymph node (MLN), and spleen (Spl), were restimulated with EBNA1 or LMP1 peptides for 6 hours, followed by surface and intracellular cytokine stain and flow cytometry. (A) Shown are the live cell counts in each culture per 100,000 cells plated; (B) the frequencies of VST cells (TNF-α/IFN-ꝩ positive) in each culture and (C) the frequency of cytotoxic CD107a / Granzyme B (GZMB) positive cells within the VST cell population. (D) Representative flow cytometry data from matched samples of an organ donor is shown to the left of compiled data showing subsets of the EBNA1 reactive cells defined by CD28 and IL-2 expression. (mean ± SEM, n = 2-4). *P < 0.05 t-test with comparison to blood.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.